15+ Chapter 7 Ionic Compounds And Metals
NCERT Solutions for Class 10 Science provides solutions to all the questions asked at the end of every chapter and the questions. Electrical resistivity also called specific electrical resistance or volume resistivity is a fundamental property of a material that measures how strongly it resists electric currentA low resistivity indicates a material that readily allows electric current.

Chapter 7 Ionic Compounds Metals Ppt Download
The electronic configurations of three elements X Y and Z are X 2 8.

. A diatomic compound or diatomic molecule is made up of two atoms that are not necessarily the same. Mercury and copper which belong to the least reactivity series are often found in the form of their sulphide ores. Potassium is the chemical element with the symbol K from Neo-Latin kalium and atomic number 19.
NCERT Solutions Class 11 Chemistry Chapter 3 Free PDF Download. Ionic compounds are the result of a metal chemically bonding with a non-metal. Table 71 Solubility Rules.
All other ionic compounds without these ions are known as salts. The fluorine atom has a nuclear charge of 9 with 2 electrons in the K shell and 7 in the L shell. Solubility of Ionic Compounds.
However these polyatomic ions form ionic compounds by combining with ions of. I Extraction of Metals of Least Reactivity. Ii Ionic compounds are solids because the particles which make up ionic compounds are held together by strong electrostatic bonds.
1115 of exam. Chemical Formula for Ionic Compounds. Valence electrons and ionic compounds.
Some compounds contain both covalent and ionic bonds. Ionic compounds are generally soluble in polar solvents such as water whereas solubility tends to decrease in non-polar solvents such as petrol gasoline etc. PH AcidBaseNeutral Color of Blue Litmus Paper Color of.
Comprehensive student-friendly answers are provided according to the latest CBSE Syllabus 2022-23 to all the in-text and exercise. Also tell what color each type of litmus paper will turn at that pH level. Ferrum and atomic number 26.
As solids they are most often. Melting and Boiling Points of Ionic Compounds. It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common.
In ionic compounds the strong electrostatic forces between ions require a high amount of energy to break. PH Scale 7 pH 14 12. The decomposition reaction exhibits first-order behavior at a quartz SiO 2 surface as suggested by the exponentially decaying plot of.
The outermost electron in the sodium atom may transfer readily to the fluorine atom. A plot of A versus t for a zero-order reaction is a straight line with a slope of k and a y-intercept of A 0Figure 1211 shows a plot of NH 3 versus t for the thermal decomposition of ammonia at the surface of two different heated solids. Resistivity is commonly represented by the Greek letter ρ The SI unit of electrical resistivity is the ohm-meter Ωm.
The later B-subgroup metals Pergamon Press Oxford pp. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O 2 breaking down in the lower atmosphere to O 2 Ozone is formed from dioxygen by the action of ultraviolet UV light and electrical discharges within the. Equilibrium Youll chart how chemical reactions change over time what causes substances to reach equilibrium and how systems react when that equilibrium is disturbed.
The atoms in polyatomic ions such as OH NO 3 NO 3 and NH 4 NH 4 are held together by polar covalent bonds. 73 Lewis Symbols and Structures. The following table can be used to help you predict which ionic compounds will be soluble in water.
Iron ˈ aɪ ə n is a chemical element with symbol Fe from Latin. Ionic compounds typically have high melting and boiling points and are hard and brittle. Nomenclature of Ionic Compounds Binary Ionic Compounds Containing a Metal and a Non-metal.
Ionic compounds do not conduct electricity in the solid-state but are good conductors in a molten state. The solubility of ionic compounds. All other ionic compounds without these ions are known as salts.
NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties are provided on this page for CBSE Class 11 students. Ii Ionic compounds are solids. Most ionic compounds are soluble in water due to the separation of ions by.
Ionic compounds containing hydrogen ions H are classified as acids and those containing hydroxide OH or oxide O 2 ions are classified as bases. All of the Group 12 metals but especially mercury tend to form covalent rather than ionic compounds The oxide of mercury in its preferred. Heating of oxides of metals to turn them into metal is known as Reduction.
191 Occurrence Preparation and Properties of Transition Metals and Their Compounds. Complete the following chart by telling whether the pH represents an acid base or neutral substance. Using the Arrhenius definitions ionic compounds containing hydrogen ions H are classified as acids and those containing hydroxide OH or oxide O 2 ions are classified as bases.
Thus the melting point and boiling point of an ionic compound are usually very high. Cinnabar HgS is the ore of mercury. 192 Coordination Chemistry of Transition Metals.
The two parts of the compounds the metal and the non-metal are known as the. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. Molecular and Ionic Compound Structure and Properties.
Usually included in this category are the group 1315 metals in periods 46. Iii With the help of a labelled diagram show the experimental set up of action of steam on a metal. Both atoms then have a complete shell but the sodium now has a.
Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula O 3It is a pale blue gas with a distinctively pungent smell. Y 2 8 7 and Z 2 8 2.
Compared with pure water the solubility of an ionic compound is less in aqueous solutions containing a common ion one also produced by dissolution of the ionic compound. A binary compound is one that is made up of two different elements. Gallium indium and thallium.
Ionic compounds are formed when. It was first isolated from potash the ashes of plants. Here Z is an ionic compound and Ionic compounds have melting point hence option b is a wrong statement.
NCERT Solutions for Class 10 Science Chapter 4 Carbon and Its Compounds helps students to understand concepts provided in the textbook in detail. Extraction of Mercury Metal. And a BCN of 6.
This is an example of a phenomenon known as the common ion effect which is a consequence of the law of mass action that may be explained using Le ChÂteliers principle. Naming salts and basic ionic compounds follows standard ionic nomenclature rules. NCERT Solutions for Class 10 Science Chapter 4 CBSE Free PDF Available.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Each element may or may not have more than one atom. 79 of exam score.
Because of this property soluble ionic compounds are referred to as electrolytes. The dissociation of soluble ionic compounds gives solutions of these compounds an interesting property. Copper glance Cu 2 S is the ore of copper.

Chapter 7 Ionic Compounds And Metals Pt Iii Youtube

Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade

Ionic Compounds And Metals Big Idea Manualzz

Chemistry Chapter 15 Ionic Bonding And Ionic Compounds
Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade

Chapter 7 Ionic Compounds Metals Ppt Download

Chapter 7 Ionic Compounds And Metals 7 1 Ion Formation Ions Are Formed When Atoms Gain Or Lose Valence Electrons To Achieve A Stable Octet Electron Configuration Ppt Download
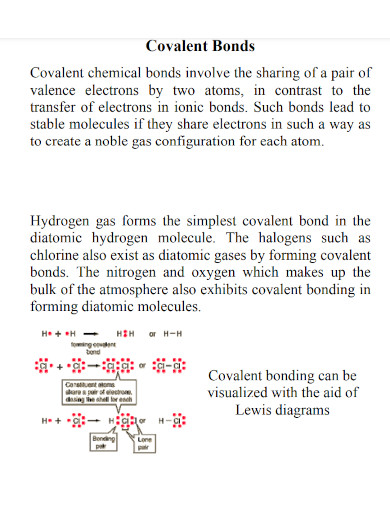
Covalent Bond Examples Pdf Examples

Chapter 7 Ionic Compounds And Metals 7 1 Ion Formation P 206 A

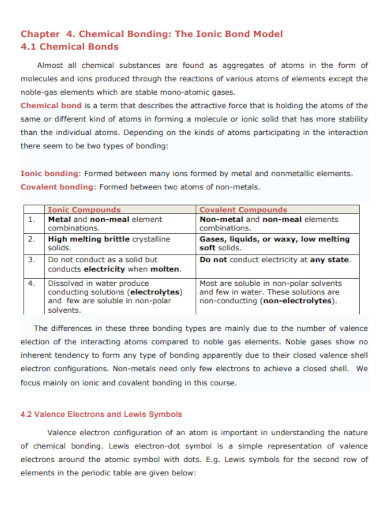
Modern Inorganic Chemistry
Ionic Bond Examples Pdf Examples

Chapter 7 Ionic Compounds And Metals Ppt Download

Chemistry Chapter 7 Ionic Compounds And Metals Flashcards Quizlet

Answers Ionic Bonding And Ionic Compounds Mutiple Choice 2 2012 07 05 Name Ionic Bonding Ionic Compounds Multiple Choice Review 1 Anions Tend To Course Hero

Chapter 7 Ionic Compounds And Metals Chemistry Matter And Change Ppt Download
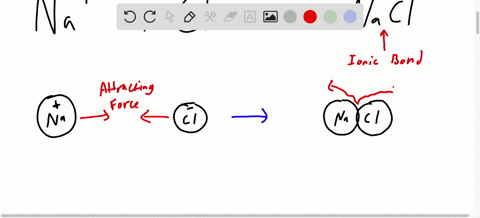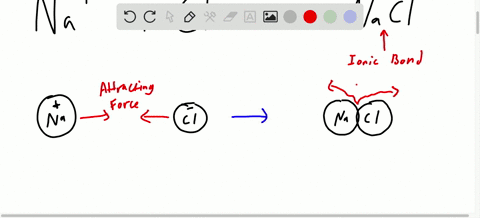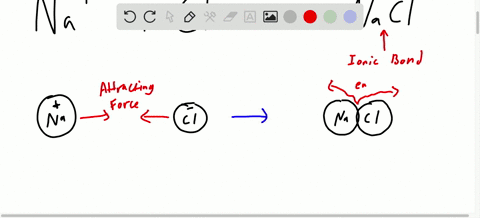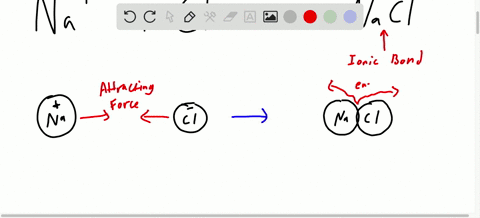
Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade
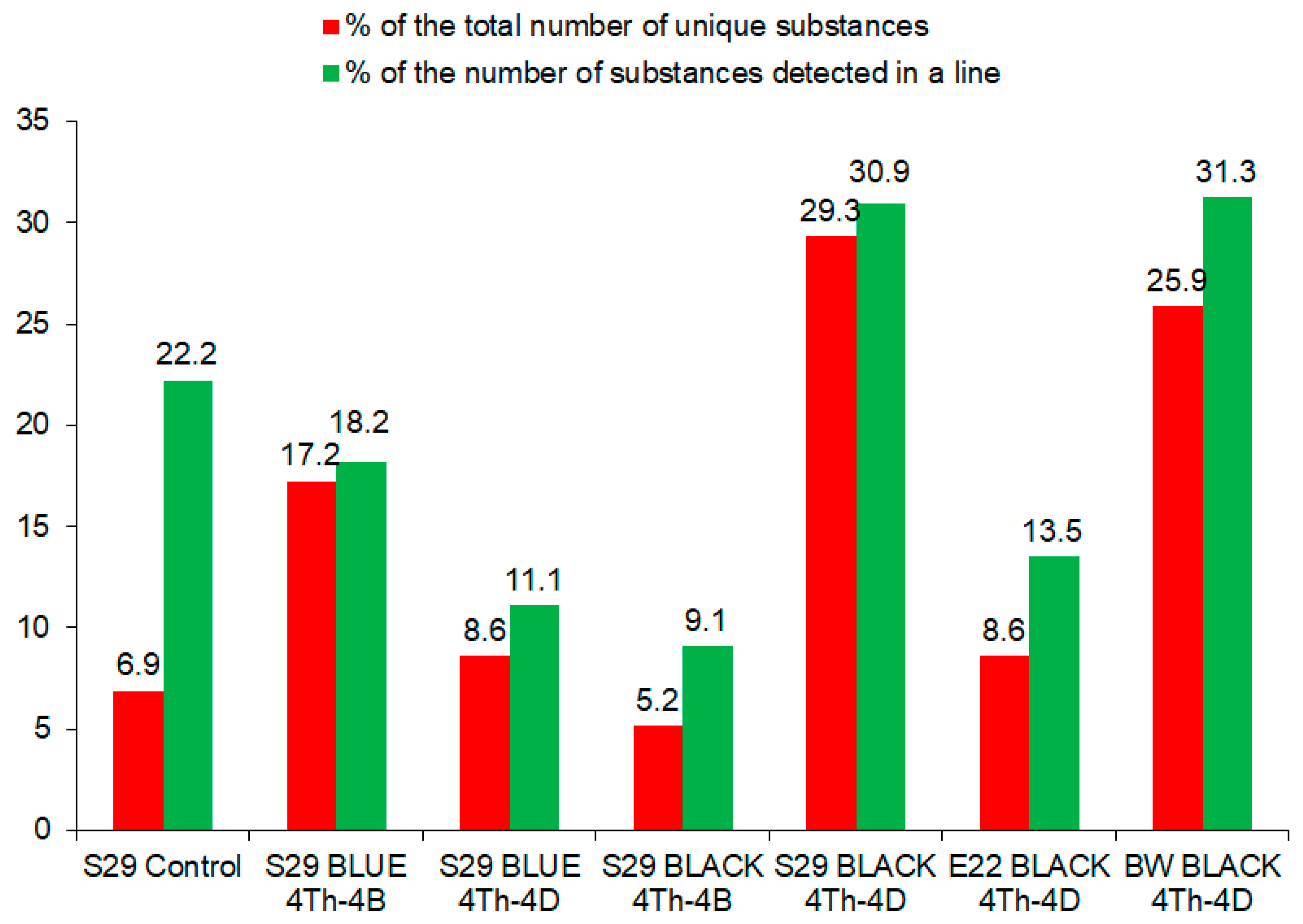
Molecules Free Full Text Phytochemical Analysis Of Phenolics Sterols And Terpenes In Colored Wheat Grains By Liquid Chromatography With Tandem Mass Spectrometry